
- +48 662 178 533
- SPF@epidermlab.pl
SPF Testing – Reliable Verification of UV Protection in Cosmetics
We provide professional SPF testing services for sunscreens, creams, and cosmetic products to verify their claimed Sun Protection Factor (SPF).
Our laboratory follows ISO 24444, ISO 24443, ISO 24442 and ISO 23675 (Double Plate) standards to deliver accurate, reproducible, and certified results recognized across Europe.
With our comprehensive SPF testing solutions, cosmetic brands can confirm product performance, meet international safety regulations, and strengthen consumer trust.
Partner with our laboratory for trusted UV protection verification and ready-to-market SPF documentation.
What Is SPF and Why SPF Testing Matters
Sun Protection Factor (SPF) indicates a product’s ability to protect skin against UVB radiation, which causes sunburn and contributes to skin cancer.
SPF testing confirms that sunscreens and cosmetics offer the declared level of UVB and UVA protection.
While SPF measures UVB defense, UVA Protection Factor (UVA-PF) evaluates protection against photoaging, pigmentation, and DNA damage.
Our laboratory performs in vivo and in vitro SPF testing according to international standards:
- ISO 24444:2019 – In vivo SPF determination
ISO 23675:2024 – In vitro SPF (Double Plate Method)
These validated methods ensure scientifically reliable and globally recognized results.
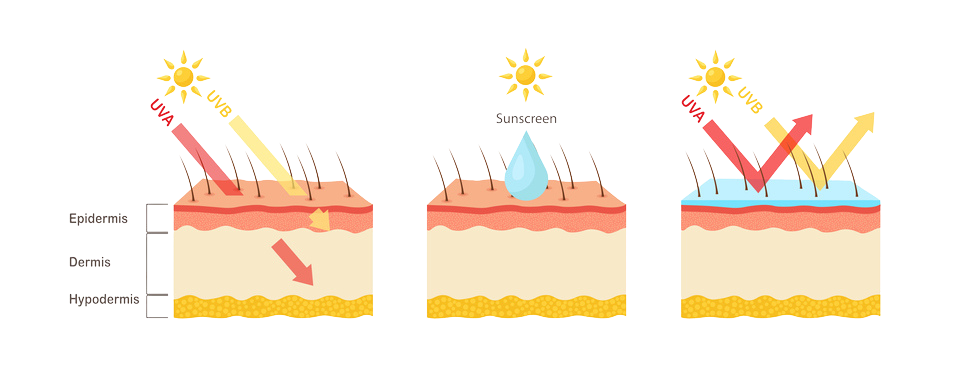
Our SPF and UV Testing Services
We offer a complete range of SPF and UV testing services designed to support your cosmetic product claims.
1. In Vivo SPF Testing (ISO 24444:2019)
Determines SPF on human skin under controlled conditions.
Evaluates protection against UVB radiation and ensures accurate labeling for sunscreens and cosmetic products.
2. In Vivo UVA Testing – PPD Method (ISO 24442:2022)
Measures UVA protection using the Persistent Pigment Darkening (PPD) method.
Essential for broad-spectrum and anti-photoaging claims.
3. In Vitro UVA-PF Testing (ISO 24443:2021)
Assesses UVA Protection Factor (UVA-PF), critical wavelength, and UVA:UVB ratio using UV spectrophotometry.
Supports UVA protection and broad-spectrum product claims.
4. In Vitro SPF Testing (ISO 23675:2024 Double Plate Method)
In vitro SPF testing is performed in accordance with ISO 23675:2024 using the Double Plate Method, ensuring high accuracy and reproducibility of results. The methodology involves the use of two types of PMMA plates to provide a reliable and representative evaluation of sunscreen SPF performance.
5. Water Resistance Testing (ISO 16217:2020, ISO 18861:2020 / FDA Guidelines)
Evaluates whether sunscreen products maintain their SPF after water immersion or sweating.
Supports marketing claims like “Water Resistant” or “Very Water Resistant.”
Additional Testing Services
We also provide anti-sand, sweat resistance, wash-off efficacy, photoallergy, and phototoxicity testing – ensuring complete UV performance and skin safety verification for cosmetic products.
Regulatory Standards and Global Compliance
All SPF and UV tests are conducted under recognized international standards, including:
- ISO 24444, ISO 24443, ISO 24442, and ISO 23675 (Double Plate)
- on the basis FDA Guidelines and AS/NZS 2604
Our laboratory ensures full regulatory documentation, suitable for EU, UK, and global cosmetic submissions.
Test Duration and Delivery Time
We understand how critical time is for product development and registration.
Our average testing timelines are:
In vivo SPF testing: around 10 working days
In vitro UVA testing: up to 10 working days
We provide fast, reliable, and certified results to help brands meet launch and compliance deadlines.
Why Choose Our SPF Testing Laboratory
- ISO-certified SPF testing methods recognized internationally
- State-of-the-art UV spectrophotometry and modern lab infrastructure
- Experienced photobiology and dermatology experts
- Fast, transparent reporting for EU and FDA documentation
Trusted by sunscreen and cosmetic brands across Europe
Our laboratory works with clients from Germany, France, Italy, Spain, Poland, the Netherlands, and the United Kingdom, offering consistent, high-quality SPF testing services across Europe.
FAQ – Frequently Asked Questions About SPF Testing
Q1: What is SPF testing?
SPF testing measures how effectively a sunscreen or cosmetic product protects against UVB and UVA radiation, according to ISO standards.
Q2: How long does an SPF test take?
SPF testing typically takes between 7 and 10 working days, depending on the selected method and sample preparation.
Q3: What is the difference between in vivo and in vitro SPF testing?
In vivo testing measures SPF directly on human skin, while in vitro testing uses UV spectrophotometry to estimate SPF on PMMA plates.
Q4: Do you perform SPF testing across Europe?
Yes. Our SPF and UV testing services are available for cosmetic and sunscreen manufacturers throughout Europe, including Germany, France, Italy, Spain, Poland, and the UK.
Contact Us for Professional SPF Testing
Looking for a reliable SPF testing laboratory in Europe?
Get in touch with our team to learn more about our ISO-certified SPF, UVA, and UV protection testing services.
E-mail: SPF@epidermlab.pl
Ask us about:
- SPF test pricing and timelines
- Sample preparation and requirements
- Water resistance and UV protection verification
Certification support for product claims
Partner with us and ensure your products meet the highest international standards for sun protection and cosmetic safety.
EpiDermLab Laboratorium badawcze s.c., Poland, 30-613 Kraków, Łowienicka 14/3, VAT-ID: PL9452271009, phone: +48 662 178 533, e-mail: SPF@epidermlab.pl
